First Law
The first law of thermodynamics is a version of the law of conservation of energy, adapted for thermodynamic processes, distinguishing two kinds of transfer of energy, as heat and as thermodynamic work, and relating them to a function of a body's state, called Internal energy.
The law of conservation of energy states that the total energy of an isolated system is constant; energy can be transformed from one form to another, but can be neither created nor destroyed which means we can't create energy and we can't destroy energy what we only can do is convert . We only can convert the energy from one form to another like ( Heat to kinetic energy , Kinetic to mechanical energy etc. )
For a thermodynamic process without transfer of matter, the first law is often formulated.
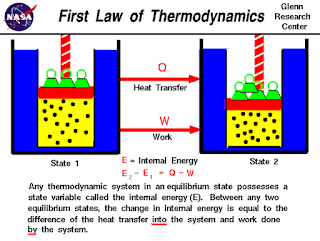
∆U = Q - W
where ΔU denotes the change in the internal energy of a closed system, Q denotes the quantity of energy supplied to the system as heat, and W denotes the amount of thermodynamic work done by the system on its surroundings. An equivalent statement is that perpetual motion machines of the first kind are impossible.
History
Scientists in the late 18th and early 19th centuries adhered to caloric theory, first proposed by Antoine Lavoisier in 1783, and further bolstered by the work of Sadi Carnot in 1824, according to the American Physical Society. Caloric theory treated heat as a kind of fluid that naturally flowed from hot to cold regions, much as water flows from high to low places. When this caloric fluid flowed from a hot to a cold region, it could be converted to kinetic energy and made to do work much as falling water could drive a water wheel. It wasn’t until Rudolph Clausius published "The Mechanical Theory of Heat" in 1879 that caloric theory was finally put to rest.
Thermodynamic systems
Energy can be divided into two parts, according to David McKee, a professor of physics at Missouri Southern State University. One is our human-scale macroscopic contribution, such as a piston moving and pushing on a system of gas. Conversely, things happen at a very tiny scale where we can’t keep track of the individual contributions.
McKee explains, “When I put two samples of metal up against each other, and the atoms are rattling around at the boundary, and two atoms bounce into each other, and one of the comes off faster than the other, I can’t keep track of it. It happens on a very small time scale and a very small distance, and it happens many, many times per second. So, we just divide all energy transfer into two groups: the stuff we’re going to keep track of, and the stuff we’re not going to keep track of. The latter of these is what we call heat.”
Thermodynamic systems are generally regarded as being open, closed or isolated. According to the University of California, Davis, an open system freely exchanges energy and matter with its surroundings; a closed system exchanges energy but not matter with its surroundings; and an isolated system does not exchange energy or matter with its surroundings. For example, a pot of boiling soup receives energy from the stove, radiates heat from the pan, and emits matter in the form of steam, which also carries away heat energy. This would be an open system. If we put a tight lid on the pot, it would still radiate heat energy, but it would no longer emit matter in the form of steam. This would be a closed system. However, if we were to pour the soup into a perfectly insulated thermos bottle and seal the lid, there would be no energy or matter going into or out of the system. This would be an isolated system.
In practice, however, perfectly isolated systems cannot exist. All systems transfer energy to their environment through radiation no matter how well insulated they are. The soup in the thermos will only stay hot for a few hours and will reach room temperature by the following day. In another example, white dwarf stars, the hot remnants of burned-out stars that no longer produce energy, can be insulated by light-years of near perfect vacuum in interstellar space, yet they will eventually cool down from several tens of thousands of degrees to near absolute zero due to energy loss through radiation. Although this process takes longer than the present age of the universe, there’s no stopping it.
Heat engines
The most common practical application of the First Law is the heat engine. Heat engines convert thermal energy into mechanical energy and vice versa. Most heat engines fall into the category of open systems. The basic principle of a heat engine exploits the relationships among heat, volume and pressure of a working fluid. This fluid is typically a gas, but in some cases it may undergo phase changes from gas to liquid and back to a gas during a cycle.
When gas is heated, it expands; however, when that gas is confined, it increases in pressure. If the bottom wall of the confinement chamber is the top of a movable piston, this pressure exerts a force on the surface of the piston causing it to move downward. This movement can then be harnessed to do work equal to the total force applied to the top of the piston times the distance that the piston moves.
There are numerous variations on the basic heat engine. For instance, steam engines rely on external combustion to heat a boiler tank containing the working fluid, typically water. The water is converted to steam, and the pressure is then used to drive a piston that converts heat energy to mechanical energy. Automobile engines, however, use internal combustion, where liquid fuel is vaporized, mixed with air and ignited inside a cylinder above a movable piston driving it downward.
Refrigerators, air conditioners and heat pumps
Refrigerators and heat pumps are heat engines that convert mechanical energy to heat. Most of these fall into the category of closed systems. When a gas is compressed, its temperature increases. This hot gas can then transfer heat to its surrounding environment. Then, when the compressed gas is allowed to expand, its temperature becomes colder than it was before it was compressed because some of its heat energy was removed during the hot cycle. This cold gas can then absorb heat energy from its environment. This is the working principal behind an air conditioner. Air conditioners don’t actually produce cold; they remove heat. The working fluid is transferred outdoors by a mechanical pump where it is heated by compression. Next, it transfers that heat to the outdoor environment, usually through an air-cooled heat exchanger. Then, it is brought back indoors, where it is allowed to expand and cool so it can absorb heat from the indoor air through another heat exchanger.
A heat pump is simply an air conditioner run in reverse. The heat from the compressed working fluid is used to warm the building. It is then transferred outside where it expands and becomes cold, thereby allowing it to absorb heat from the outside air, which even in winter is usually warmer than the cold working fluid.
Geothermal or ground-source air conditioning and heat pump systems use long U-shaped tubes in deep wells or an array of horizontal tubes buried in a large area through which the working fluid is circulated, and heat is transferred to or from the earth. Other systems use rivers or ocean water to heat or cool the working fluid.
Source : livescience , wikipedia
Thanks for reading . I hope you understand this the next part will come soon .
If you have any question then comment down .
Don't forget to follow me .
Facbook Link
Instagram Link
Twitter Link

Post a Comment